
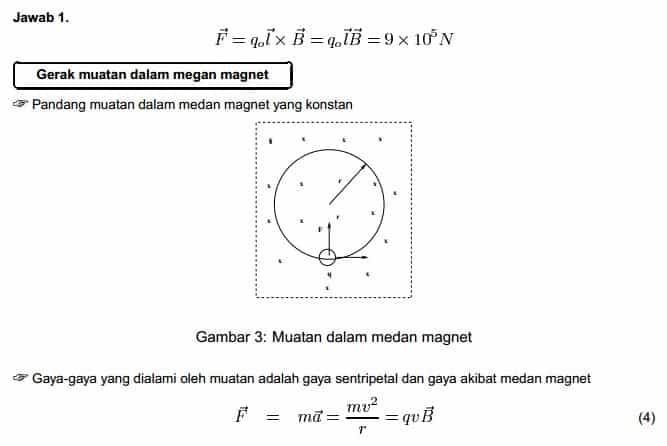
Konsep-konsep fisika tersusun sederhana yang mana satu sama lain konsep membentuk jalinan yang utuh sehingga mampu menjelaskan setiap fenomena alam. Oleh karena itu langkah awal adalah menguasai konsep dasar fisika. Ibarat fisika adalah seorang penari balet yang menari di alam semesta sepanjang jagad makro sampai jagad mikro, maka matematika adalah pakaian yag dikenakan penari itu. Belajar fisika tetap memerlukan matematika. Materi fisika SMA/MA/SMK sebenarnya merupakan pendalaman dan perluasan materi fisika SMP dengan analisa yang lebih mendalam.

RoentgenĬalled it "X " to indicate it was an unknown type of The photograph electrified the general publicĪnd aroused great scientific interest in the new form of radiation. X-ray photograph of his wife's hand which clearly revealed her wedding ring and Roentgen placed various objects between the tube and the screen, and the screen Roentgen assumed this has blocked most of the radiation. In reaction to electromagnetic radiation, but Roentgen's tube was surrounded by Response in itself was not so surprising. Screen in his lab started to glow when the electron beam was turned on. Wilhelm Conrad Roentgen, a German scientist who found them quite byĪccident when experimenting with electron beams in a gas discharge tube. X-rays were first observed and documented in 1895 by Contours of constant probability, often referred to as “clouds” may beĭrawn around the nucleus of an atom to conceptualize where the electron mightīe located with the most probability (Figure 8).Įlectromagnetic spectrum. Located somewhere within a region of space, but with their exact positions being For instance, electrons may be considered to be Predictions of conjugate variables, such as position and momentum, Contrary to classical mechanics, one can never make simultaneous Of finding an electron in a particular region around the nucleus at a For example, it allows one to compute the probability This abstract mathematical object allows for the calculation of probabilities Generally, elements of a complex vector space.
(sometimes referred to as orbital in the case of atomic electrons), and more In theįormalism of quantum mechanics, the state of a system at a given time is described Laws of motion and by Maxwell's laws of classical electromagnetism. Staying in its orbital, which could not be explained by Newton's The quantum theory of the atom was developed as an explanation for the electron's This branch of physics was initially developed to provide a betterĮxplanation of the atom, especially the spectra of light emitted by To the branch of physics that deals with atomic and subatomic systems That waves have discrete energy packets (called quanta) that behave in a manner similar to particles led Physical quantities, such as the energy of an atom at rest. Mechanics, it refers to a discrete unit that quantum theory assigns to certain The effects of quantum mechanics are typically not observable on macroscopic scales, but become evident at the atomicĬame from the Latin word which means "what quantity". Quantum theoryĭescriptions for many previously unexplained phenomena such Quantum mechanics is a fundamental branch of physics with wide applications. Mechanics is the study of mechanical systems whoseĭimensions are close to or below the atomic scale, such as molecules, atoms,Įlectrons, protons and other subatomic particles. Thousand times greater than for the outer electrons, the The inner electrons, those closer to the nucleus, areĪnother particle, an inner electron may be excited.Įlectron returns to the inner orbit, the excess energy TheĬontains only lines associated with transitions
Most of the atoms are in the ground state. This is called the absorption spectrum of the Shows gaps corresponding to the absorption ofĪtoms. Passed through a group of atoms of a given species, Light at other frequencies is not absorbed. To one of its higher allowed energy states.Īt the specific transition frequencies, given byĮquation 1. Must be such that each photon has just the right
Can also be excited to a higher level by light at a


 0 kommentar(er)
0 kommentar(er)
